Saving Heating Bills by Learning to Apply Thermodynamics
With soaring power bills, heating in winter is fast becoming a luxury. In cities like Sydney or Melbourne where winter low temperature hovers at just above freezing, room heating can become an optional extra. It is also very unlikely that pipes will freeze in winter in Sydney.
By learning a little about Thermodynamics you can learn the science behind saving on heating bills this winter.
Thermodynamics is the study of heat and temperature and its relation with energy and work. Most of us would have learnt bits and pieces in Science or Physics class while in school. Here are 3 basic concepts.
1. Heat moves by 3 ways - Conduction, Convection and Radiation.Thermodynamics is the study of heat and temperature and its relation with energy and work. Most of us would have learnt bits and pieces in Science or Physics class while in school. Here are 3 basic concepts.
Conduction
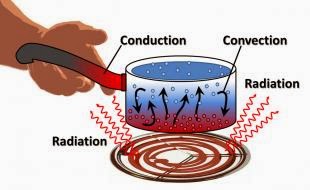 |
| (Source: Climate Science Investigations) |
Conduction is the movement of heat without the movement of particles. A good example would be heat moving from the bottom of your pot when you heat it to the top of the pot. The pot material itself does not move, but the heat moves. Conduction always happen from down a gradient - meaning from a hotter place to a colder place.
Convection
Convection is the movement of heat using the movement of particles - particular in gases and liquids. A good example would be a heater fan blowing hot air across the room. Each air particle moves from the fan to the end of the room. Convection also can happen without assistance by the natural random movement of particles; or for gases as the particles are heated it gets less dense and rise (ideal gas law: PV=nRT).
Radiation
Radiation is the movement of heat through electromagnetic waves emitted from a surface. An example is a radiation heater. Heat is emitted by the heater and moves into the surroundings through radiation. In a sense, the more surface there is, the more heat is radiated, hence why radiators have corrugated surface.
2. Laws of Thermodynamics
There are 4 laws of thermodynamics. For the purpose of this article, the law I would focus on is the 1st Law of Thermodynamics which is also known as the Law of Conversation. Energy cannot be created from no where or destroyed into oblivion. If you use a heater to heat a room, all the heat energy emitted from the heater is transferred into the room. Heat energy can be loss to outside the room or converted to another form of energy but cannot be destroyed.
3. Thermal Conductivity
Different materials have different thermal conductivity. As shown in the diagram, the lower the thermal conductivity, the better material is as an insulator. The higher the thermal conductivity, the better the conductor. Hence we see that gases, such as air are a great thermal insulator. In practical sense, air trapping materials such as puffy down jackets help keep us warm.
Thermal Conductivity of Different Materials (Source: Fundamentals of Heat and Mass Transfer)
So how do we apply Thermodynamics to saving on our heating bills? To find out more go to my next post.
References
Fundamentals of Heat and Mass Transfer, F Incropera & D DeWitt
Related Posts
Ways to Save on Heating this Winter
To subscribe to my blog, please Like on our Facebook page.
To support my blog, please click on one of my sponsor's advertised links :)



Comments
Post a Comment